Full Text View
Volume 21 Issue 1 (January 2011)
GSA Today
Article, pp. 4-9 | Abstract | PDF (3.7MB)
Microbial communities in fluid inclusions and long-term survival in halite
| Table of Contents |
|---|
|
Search GoogleScholar for
Search GSA Today |
Abstract
Fluid inclusions in modern and ancient buried halite from Death Valley and Saline Valley, California, USA, contain an ecosystem of “salt-loving” (halophilic) prokaryotes and eukaryotes, some of which are alive. Prokaryotes may survive inside fluid inclusions for tens of thousands of years using carbon and other metabolites supplied by the trapped microbial community, most notably the single-celled alga Dunaliella, an important primary producer in hypersaline systems. Deeper understanding of the long-term survival of prokaryotes in fluid inclusions will complement studies that further explore microbial life on Earth and elsewhere in the solar system, where materials that potentially harbor microorganisms are millions and even billions of years old.
*E-mail:
**Now at Dept. of Geology and Geophysics, University of Hawai'i at M¯anoa, Honolulu, Hawai'i 96822, USA
Manuscript received 19 Sept. 2009; accepted 25 Apr. 2010
DOI: 10.1130/GSATG81A.1
INTRODUCTION
Microbes are known to exist in subsurface habitats, such as sub-seafloor sediments and continental and oceanic crust, to depths of up to ~3 km (Parkes et al., 2000; Kerr, 2002; Lin et al., 2006; Onstott et al., 2006). Prokaryotes (single-celled organisms lacking a nucleus and other membrane-bound specialized structures) in these subsurface environments live in water within sediment pores and rock fractures. Most are heterotrophic and depend upon preexisting organic matter around them for metabolism, but some are autotrophic and can use non-photosynthetically derived energy sources (Lin et al., 2006). Other prokaryotes that live in Earth's subsurface under such so-called “extreme” conditions have been found in ice as old as 120 ka from Antarctica, Greenland, and mountain glaciers, and in perma-frost, perhaps as old as 8 Ma (Christner et al., 2000; Miteva et al., 2004, 2005; Bidle et al., 2007; Johnson et al., 2007). Collectively, these discoveries have extended the realm of the biosphere into Earth's crust and have given hope for finding life beneath the surface of other planets, moons, asteroids, and comets of our solar system where present surface conditions are inhospitable.
The world's “oldest living organisms” come from another subsurface setting, buried salt deposits. Over the past 50 years, a series of papers have claimed long-term survival of prokaryotes (Bacteria and Archaea) in these deposits, in some cases for >250 m.y. (Reiser and Tasch, 1960; Dombrowski, 1963; Norton and Grant, 1988; Grant et al., 1998; Stan-Lotter et al., 1999; McGenity et al., 2000; Vreeland et al., 2000, 2007; Radax et al., 2001; Mormile et al., 2003; Schubert et al., 2010a). Prokaryotes in ancient salt deposits also apparently survived in water, but in this case were confined to brine-filled “fluid inclusions” in the halite itself, isolated from surrounding pore- and fracture-filling waters.
Reports of extreme microbe longevity in salt are controversial. The well-known Permian bacterium from the Waste Isolation Pilot Plant (WIPP) site, Salado Formation, New Mexico, USA (Vreeland et al., 2000), for example, comes from a brine inclusion within a large, diagenetically formed halite crystal. That brine inclusion could have been trapped after the Permian during burial cementation and recrystallization processes (Hazen and Roedder, 2001). Later study of those fluid inclusions, however, shows that they most likely contain evaporated Permian seawater, which supports their 250 Ma age and the antiquity of the trapped bacterium (Satterfield et al., 2005). The strongest criticism of the antiquity of prokaryotes recovered from ancient salt deposits has come from the biological science community, which maintains that deoxyribonucleic acid (DNA) should degrade over time scales far shorter than 250 m.y. in the absence of a repair mechanism (Willerslev et al., 2004; Hebsgaard et al., 2005; Willerslev and Hebsgaard, 2005). In addition, DNA from the Permian bacterium is nearly identical to a modern bacterium, Virgibacillus marismortui, sampled from the Dead Sea (Arahal et al., 1999, 2000), which suggests to some that the Permian bacterium is a laboratory contaminant (Graur and Pupko, 2001).
Other reputed ancient Archaea occur in bedded halite with primary growth textures and banded arrays of primary fluid inclusions parallel to crystal growth faces, indicating that the inclusions were trapped during growth of halite from surface brines (Mormile et al., 2003; Vreeland et al., 2007; Schubert et al., 2009a, 2010a). It is now certain that some ancient bedded halite, and the included brines and microorganisms, can remain undisturbed for millions of years (Lowenstein et al., 2001). The problem confronting all studies of prokaryotes trapped in fluid inclusions from ancient halite is understanding how these microorganisms survive for prolonged periods and how they obtain energy to perform necessary functions, such as repair of damaged DNA.
Here we examine microorganisms trapped in fluid inclusions in halite, summarizing results from modern environments (Saline Valley, California, USA) and buried deposits up to 100 ka from Death Valley, California, USA (Schubert et al., 2009a, 2009b, 2010a, 2010b). We also present new, unpublished information from the subsurface salts of Saline Valley, which are up to 150 ka. These modern and Pleistocene deposits contain significant numbers of prokaryotes in fluid inclusions, a small number of which are clearly alive. Microscopy has revealed a remarkable “ecosystem” within fluid inclusions, composed of “salt-loving” (halophilic) prokaryotes and eukaryotes (complex cells containing a nucleus and specialized structures, such as chloroplasts) that may hold key information about long-term survival. We hypothesize that prokaryotes survive inside fluid inclusions for prolonged periods using carbon and other metabolites supplied by members of the trapped microbial community, most notably the single-celled alga Dunaliella, an important primary producer in hypersaline systems.
Halophilic Microorganisms in Modern Hypersaline Systems
The starting point for evaluating long-term survival of micro-organisms in fluid inclusions in salt is to examine modern evaporite systems and the processes by which organisms are preserved in halite there. We illustrate a typical hypersaline environment, Saline Valley, where, under certain conditions, surface brines host prolific numbers of halophilic microorganisms. Saline Valley is a closed-basin saline pan in eastern California that contains surface brines up to 0.5 m deep, fed by groundwaters (Figs. 1 and 2A) (Hardie, 1968; Howe, 1998). A bloom of planktonic halophiles, developed in March 2004, contained one type of photosynthetic autotroph, the single-celled alga Dunaliella, and many heterotrophs (prokaryotic Archaea and Bacteria, that thrived in bright red brines at salinities of 26%–30%, seven to eight and a half times more concentrated than seawater (Fig. 2B). The pink/red brine color is due to the carotenoids (organic pigments, including β-carotene used by microorganisms for protection from ultraviolet radiation) in halophilic Archaea and Bacteria and Dunaliella (Teller, 1987; Pedrós-Alió et al., 2000; Oren and Rodríguez-Valera, 2001; Oren, 2002b). Wet mounts prepared from Saline Valley brines contained rod- and coccoid-shaped prokaryotes and larger spherical and ellipsoid-shaped cells of Dunaliella, some of which were motile one year after collection (Figs. 2C and 2D).
|
Map of Death Valley and Saline Valley, California, USA, with locations of cores DV93-1 and SV-4A; modified from Schubert et al. (2009a). |
|
Saline Valley, California, USA, March 2004. (A) Saline pan and surrounding mudflats, with surficial salt crust (white) and shallow saline lake in foreground. (B) Halophile bloom in saline lake. (C) Photomicrograph of wet mount slide prepared from Saline Valley brine (Oct. 2005), with rod- and spherical (cocci) shaped microbes distinct from diamond-shaped crystal of glauberite (CaSO4 • Na2SO4). Scale bar is 10 μm. Modified from Schubert et al. (2009a). (D) Photomicrograph of wet mount slide prepared from Saline Valley brine, with spherical green cells of Dunaliella. Scale bar is 10 μm. (E) Large rafts (up to 1 m) of laterally linked halite crystals on the brine surface and halite chevrons crystallizing at the brine bottom. (F) Cross section of halite crust formed in 2004, pink from trapped microorganisms. Small divisions on ruler are millimeters. (G) Thin-section photomicrograph of halite crust shown in F. Vertically oriented halite crystals grew upward from the saline lake bottom. Fluid inclusion bands (gray) in some halites outline primary crystal growth directions. Scale bar is 10 mm. |
When surface brines from Saline Valley evaporated to salinities greater than ~30% during March 2004, halite saturation was reached and halite crystals nucleated at the air-brine interface, forming floating masses of linked crystal rafts; vertically oriented crystals also grew off the brine bottom (Fig. 2E). The halite crust formed by these processes contained large numbers of brine inclusions trapped during crystal growth, and the salt crust was pink because microbes from the water column were incorporated into the halite inclusions (Figs. 2F and 2G). Individual fluid inclusions housed a community of prokaryotes and Dunaliella, the same shape and size as observed in Saline Valley brines (Fig. 3). Microscopic study of >1000 brine inclusions from 10 halite-crust crystals showed that >20% contained prokaryotes (Schubert et al., 2009a). The calculated prokaryote abundance of 6 × 108 microbes/mL of inclusion brine is similar to that reported from red halophile-rich brines in many modern settings (Larsen, 1980; Teller, 1987; Oren, 2002a, 2002b; Pedrós-Alió, 2004). This means that one halite cube from Saline Valley, 1 cm per side, with a typical volume of fluid inclusions of 1% (Roedder and Bassett, 1981) contains six million trapped microbes.
|
Photomicrographs of fluid inclusions in halite, collected in Saline Valley California, USA, in 2004 and 2005. (A) Horizontal band rich in rectangular prism-shaped brine inclusions, surrounded above and below by bands containing fewer inclusions. Scale bar is 100 μm. (B) Tubular fluid inclusions and evenly distributed cubic and rectangular prism-shaped inclusions. Arrows point to inclusions shown at higher magnification in C and D. Scale bar is 100 μm. (C) and (D) Fluid inclusions with rod- and coccoid-shaped prokaryotes. Scale bars are 10 μm. (E) and (F) Portions of large fluid inclusions in halite with ellipsoidal and spherical cells of Dunaliella and numerous smaller prokaryotes. Scale bars are 5 μm. |
Experiments show that prokaryotes (Archaea and Bacteria) trapped in fluid inclusions in halite from Saline Valley for up to 15 years can be readily cultured when placed in nutrient-rich media. These results are consistent with data from laboratory experiments and other modern surface halite deposits, all of which show that prokaryotes can remain alive inside fluid inclusions in halite for many years (Norton and Grant, 1988; Grant et al., 1998; McGenity et al., 2000; Mormile et al., 2003; Adamski et al., 2006; Fendrihan et al., 2006). The next step is to ascertain if prokaryotes remain alive in fluid inclusions following burial.
Halophilic Microorganisms in Buried Pleistocene Salt
Borehole cores from Death Valley and Saline Valley, composed of interbedded salt and mud, provide ideal materials for assessing the fate of microbial communities trapped in fluid inclusions in halite in the subsurface for periods of up to 150 k.y. Fig. 4). The cored sediments contain a dated record of Pleistocene paleoenvironments that varied from saline pans and dry mudflats to deep, perennial lakes (Li et al., 1996; Howe, 1998; Lowenstein et al., 1999). Evaporites accumulated in two settings: (1) bedded halite with abundant primary growth textures formed in perennial saline lakes (i.e., Great Salt Lake, Utah, USA); and (2) massive halite formed in salt pans (i.e., Badwater Basin, Death Valley, USA) (Li et al., 1996; Lowenstein et al., 1999). Microorganisms in fluid inclusions were almost exclusively found in halites deposited in perennial saline lakes in Death Valley (ca. 10–35 ka) and Saline Valley (ca. 20 ka, 75 ka, and 150 ka). Some of these halites have prokaryotes in fluid inclusions comparable in abundance to those found in modern halites formed during the 2004 Saline Valley halophile bloom, which suggests that ancient saline lakes of Death Valley and Saline Valley were at times teeming with microorganisms (Schubert et al., 2009a).
|
Stratigraphic columns, of cores DV93-1 and SV-4A showing sediment types, uranium-series ages, and paleoenvironments. Modified from Howe (1998) and Lowenstein et al. (1999). Note depths (arrows) where samples were taken for culturing experiments: green—unsuccessful; red—successful. |
Dunaliella cells trapped in fluid inclusions up to 150 ka may appear virtually the same as those from modern halite (compare Fig. 5A to Figs. 3E and 3F). Remarkably, some ancient Dunaliella cells contain a cup-shaped chloroplast and are green and orange, which suggests preservation of pigments, including carotenoids and chlorophyll (Fig. 5B) (Schubert et al., 2010b). Other ancient Dunaliella cells, particularly in fluid inclusions in halite from the Saline Valley core, form a “stew” in various stages of disintegration, with cell coats separated from cell contents (Fig. 5F).
|
Photomicrographs of fluid inclusions in ancient halite from Saline Valley and Death Valley (Calif., USA) cores. (A) Dunaliella cell (left) and miniaturized prokaryotes (circled), in irregularly shaped fluid inclusion, Saline Valley core, 93 m, 150 ka. (B) Light green and orange Dunaliella cells suggest preservation of chlorophyll and β-carotene, Death Valley core, 17.8 m, 34 ka. Modified from Schubert et al. (2010b). (C) Miniaturized prokaryotes in cubic fluid inclusion, Death Valley core 16.5 m, 31 ka. Modified from Schubert et al. (2009a). (D) Portion of large fluid inclusion containing yellow-green Dunaliella cells and two cells coated with outward radiating crystals of β-carotene (brown). Miniaturized prokaryote is circled. Death Valley core 15.7 m, 29 ka. (E) Portion of fluid inclusion showing Dunaliella cells heavily coated with crystalline β-carotene, Death Valley core 15.7 m, 29 ka. Arrow shows the boundary between the fluid inclusion and the host halite crystal. (F) Dunaliella cells in various stages of degradation within a large fluid inclusion, Saline Valley core, 44 m, ca. 70 ka. Arrow shows ruptured glycocalyx (cell coat) of one Dunaliella cell. |
Prokaryotes found in buried halites (>10 ka) appear quite different from those trapped in fluid inclusions in modern halite. Ancient prokaryotes are coccoid-shaped and “miniaturized,” with cell diameters <1 μm (Figs. 5A, 5C, and 5D), much smaller than the straight or curved rods (1–10 μm long, ~0.5–1 μm wide) and coccoid-shaped prokaryotes (typically ~1 μm diameter), of their surface counterparts (Figs. 3C and 3D). The differences in size and shape between modern and ancient prokaryotes trapped in fluid inclusions resemble the “starvation-survival” forms reported for prokaryotes living in soils and in the ocean (Novitsky and Morita, 1976; Morita, 1982, 1997; Grant et al., 1998). It is widely known that some prokaryotes living under nutrient-poor conditions adjust by changing shape—that is, “rounding” from rod-shaped to coccoid-shaped, and reducing their size (Kjelleberg et al., 1983). We postulate that once trapped inside fluid inclusions for long periods of time, prokaryotes resort to starvation-survival strategies, but the timing and triggering mechanisms are not known. Trapping of halophilic Archaea in nutrient-free fluid inclusions in experimentally grown halite also led to rounding and cell-size reduction over periods of weeks to years (Norton and Grant, 1988; Fendrihan and Stan-Lotter, 2004), but more research on starvation-survival of prokaryotes in fluid inclusions is clearly needed.
Long-term survival of miniaturized prokaryotes in fluid inclusions in buried halite from Death Valley and Saline Valley was tested with culturing experiments designed to grow halophilic microorganisms. One procedure used previously by microbiologists involves surface sterilization of a halite crystal, followed by dissolution of that crystal in a liquid medium composed of Na+, Cl-, inorganic nutrients, and a carbon source (Vreeland et al., 2007; Schubert et al., 2009b, 2010a). During the dissolution process, the Na+, Cl-, inclusion brines, and trapped microorganisms mixed with the growth medium. Incubation under aerobic conditions for periods of up to 90 days led to the growth of cultures from five halite crystals (13.0–17.9 m; 22 ka to 34 ka) out of ~900 tested from the Death Valley core (Fig. 4) (Schubert et al., 2009b, 2010a). For unknown reasons, no prokaryotes were cultured from >500 halite crystals (12 intervals between 34 and 93 m) up to 150 ka from the Saline Valley core. It is clear from these experiments that cultivation of prokaryotes sampled from fluid inclusions in halite between 10 ka and 150 ka is rare, occurring in only 0.4% of the crystals tested. These results, coupled with the large number of cells observed in situ within fluid inclusions (Fig. 5), suggest that most ancient prokaryotes in halite are dead or viable but nonculturable, or that our culturing conditions were simply not suitable (Amann et al., 1995). Nevertheless, the DNA from the five cultured organisms from the Death Valley core shows that they are halophilic Archaea from the genera Haloterrigena, Natronomonas, and Halorubrum, all organisms expected in hypersaline lakes (Schubert et al., 2009b, 2010a).
Mechanism for Long-Term Survival of Prokaryotes in Fluid Inclusions
All Archaea from the Death Valley core we have cultured so far came from one stratigraphic interval (Fig. 4) in which prokaryotes and Dunaliella were observed in situ within fluid inclusions. Closer inspection of those fluid inclusions, coupled with what is known about the ecology of modern hypersaline systems, has led us to hypothesize a mechanism that may allow prokaryotes to survive inside fluid inclusions for millennia.
Modern hypersaline environments near halite saturation contain a productive but relatively simple community of planktonic microorganisms, with Dunaliella the only primary producer and a number of different heterotrophic Archaea and subordinate Bacteria (Pedrós-Alió et al., 2000; Elevi Bardavid et al., 2008). Much is still unknown about the prokaryotes because these ecosystems are dominated by nonculturable microbes (Oren, 2002b). Regardless, it has long been postulated that the heterotrophic community of prokaryotes in these extreme environments obtains most of its carbon requirements from glycerol, a sugar alcohol with the chemical formula C3H5(OH)3 (Borowitzka, 1981; Elevi Bardavid et al., 2008). Glycerol is produced in large quantities by Dunaliella because it is used for osmoregulation to reduce the chemical potential gradient of H2O and to prevent the loss of water from cells. In fact, Dunaliella may have concentrations of 6–7 M glycerol in their cytoplasm to counteract the chemical gradients (Elevi Bardavid et al., 2008). This glycerol apparently leaks out of healthy Dunaliella cells into surrounding brines or may enter brines following death and disintegration (lysis) of the cells (Elevi Bardavid et al., 2008). In either case, glycerol constitutes a major source of carbon for the prokaryote community in modern hypersaline systems (Borowitzka, 1981; Elevi Bardavid et al., 2008). We hypothesize that the same relationships hold true inside fluid inclusions and that glycerol and other metabolites leaked out of Dunaliella cells have supplied associated heterotrophic prokaryotes with the carbon and energy sources required for their prolonged survival. Close inspection shows that Dunaliella commonly occur with prokaryotes in fluid inclusions (Figs. 5A and 5D). Some Dunaliella are in various stages of disintegration, indicating leakage of biomaterials, including glycerol, from cells into the surrounding brine (Fig. 5F). Other Dunaliella contain a crust of crystalline β-carotene on their exteriors (Figs. 5B, 5D, and 5E) (Schubert et al., 2009b, 2010b). β-carotene is produced by certain species of Dunaliella, so finding it precipitated outside the cell is direct evidence that intracellular materials have leaked into fluid inclusions. Solid crystals apparently formed as a crust on Dunaliella cells because β-carotene is insoluble in water and thus crystallized when extruded from cells. Glycerol, however, is soluble in water and thus would be completely dissolved in fluid inclusion brines, where it would be available for heterotrophic micro-organisms. Support for our “glycerol” hypothesis comes from the five halophilic Archaea revived from fluid inclusions in Death Valley halite, all of which were cultured in media containing glycerol as a carbon source (Schubert et al., 2009b, 2010a). Two of these strains grew in media containing glycerol as the only carbon source; the other three are yet to be tested.
Conclusions
Although we are beginning to understand the community of microorganisms inside modern and ancient fluid inclusions, much more needs to be learned about how they survive. Miniaturized prokaryote cells suggest starvation-survival, despite the availability of carbon. We do not know why prokaryotes in fluid inclusions miniaturize, what factors trigger miniaturization, and what functions miniaturized cells are able to perform in fluid inclusions (e.g., repair of DNA and cell membranes) (Grant et al., 1998; Johnson et al., 2007). Alternatively, prokaryotes may form spores and survive for long periods in a dormant state, as has been claimed for the bacterium cultured from the Permian fluid inclusion by Vreeland et al. (2000). But none of the halophilic Archaea cultured from the Death Valley core formed endospores, nor do any Archaea. We thus need to learn more about long-term survival of spore-forming prokaryotes as well as miniaturized forms trapped in fluid inclusions. Such knowledge will be vital as studies further explore deep life on Earth and elsewhere in the solar system, where materials that potentially harbor microorganisms are millions and even billions of years old.
Acknowledgments
Many thanks to Matthew Parker for guidance on PCR amplification and DNA sequencing, Jürgen Polle for teaching us about Dunaliella, Aharon Oren for insights on halophiles, Richard Ku and Shangde Luo for age dating the salt cores, Robert Demicco for his review of this manuscript, and Russell Vreeland for getting us started on the problem. Current and former graduate students Kathy Benison, Chris Brown, Jianren Li, Laura Howe, Sean Brennan, Cindy Satterfield, Kathryn Gragg, Yaicha Winters, Deidre LaClair, and Elliot Jagniecki contributed to the ideas developed in this paper. This project was supported by U.S. National Science Foundation Biogeosciences Grants EAR-0433802 and EAR-1024692.
REFERENCES CITED
- Adamski, J.C., Roberts, J.A., and Goldstein, R.H., 2006, Entrapment of bacteria in fluid inclusions in laboratory-grown halite: Astrobiology, v. 6, p. 552–562.
- Amann, R.I., Ludwig, W., and Schleifer, K.-H., 1995, Phylogenetic identification and in situ detection of individual microbial cells without cultivation: Microbiological Reviews, v. 59, p 143–169.
- Arahal, D.R., Márquez, M.C., Volcani, B.E., Schleifer, K.H., and Ventosa, A., 1999, Bacillus marismortui sp. nov., a new moderately halophilic species from the Dead Sea: International Journal of Systematic Bacteriology, v. 49, p 521–530.
- Arahal, D.R., Márquez, M.C., Volcani, B.E., Schleifer, K.H., and Ventosa, A., 2000, Reclassification of Bacillus marismortui as Salibacillus marismortui comb. nov.: International Journal of Systematic Bacteriology, v. 50, p 1501–1503.
- Bidle, K.D., Lee, S., Marchant, D.R., and Falkowski, P.G., 2007, Fossil genes and microbes in the oldest ice on Earth: Proceedings of the National Academy of Sciences, v. 104, p. 13,455–13,460.
- Borowitzka, L.J., 1981, The microflora: Adaptations to life in extremely saline lakes: Hydrobiologia, v. 81, p. 33–46.
- Christner, B.C., Mosley-Thompson, E., Thompson, L.G., and Zagorodnov, V., 2000, Recovery and identification of viable bacteria immured in glacial ice: Icarus, v. 144, p. 479–485.
- Dombrowski, H., 1963, Bacteria from Paleozoic salt deposits: Annals of the New York Academy of Sciences, v. 108, p 453–460.
- Elevi Bardavid, R., Khristo, P., and Oren, A., 2008, Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds: Extremophiles, v. 12, p. 5–14.
- Fendrihan, S., and Stan-Lotter, H., 2004, Survival of halobacteria in fluid inclusions as a model of possible biotic survival in Martian halite, in Teodorescu, H.N., and Griebel, H.S., eds., Mars and Planetary Science and Technology: Iasi, Romania, Performantica Press, p. 9–18.
- Fendrihan, S., Legat, A., Pfaffenhuemer, M., Gruber, C., Weidler, G., Gerbl, F., and Stan-Lotter, H., 2006, Extremely halophilic archaea and the issue of long-term microbial survival: Reviews in Environmental Science and Biotechnology, v. 5, p. 203–218.
- Grant, W.D., Gemmell, R.T., and McGenity, T.J., 1998, Halobacteria: The evidence for longevity: Extremophiles, v. 2, p. 279–287.
- Graur, D., and Pupko, T., 2001, The Permian bacterium that isn't: Molecular Biology and Evolution, v. 18, p. 1143–1146.
- Hardie, L.A., 1968, The origin of the Recent non-marine evaporite deposit of Saline Valley, Inyo County, California: Geochimica et Cosmochimica Acta, v. 32, p. 1279–1301.
- Hazen, R.M., and Roedder, E., 2001, Biogeology: How old are bacteria from the Permian age?: Nature, v. 411, p. 155–155.
- Hebsgaard, M.B., Phillips, M.J., and Willerslev, E., 2005, Geologically ancient DNA: Fact or artefact?: Trends in Microbiology, v. 13, p. 212–220.
- Howe, L.K., 1998, 150 ka paleoclimate history and chemical evolution of evaporites, Saline Valley, California [M.A. thesis]: Binghamton, State University of New York, 174 p.
- Johnson, S.S., Hebsgaard, M.B., Christensen, T.R., Mastepanov, M., Nielsen, R., Munch, K., Brand, T., Gilbert, M.T., Zuber, M.T., Bunce, M., Ronn, R., Gilichinsky, D., Froese, D., and Willerslev, E., 2007, Ancient bacteria show evidence of DNA repair: Proceedings of the National Academy of Sciences, v. 104, p. 14,401–14,405.
- Kerr, R.A., 2002, Deep life in the slow, slow, lane: Science, v. 296, p. 1056–1058.
- Kjelleberg, S., Humphrey, B.A., and Marshall, K.C., 1983. Initial phases of starvation and activity of bacteria at surfaces: Applied and Environmental Microbiology, v. 46, p. 978–984.
- Larsen, H., 1980, Ecology of hypersaline environments, in Nissenbaum, A., ed., Hypersaline brines and evaporitic environments: Developments in Sedimentology 28, p. 23–39.
- Li, J., Lowenstein, T.K., Brown, C.B., Ku, T.-L., and Luo, S., 1996, A 100 ka record of water tables and paleoclimates from salt cores, Death Valley, California: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 123, p. 179–203.
- Lin, L.H., Wang, P.-L., Rumble, D., Lippmann-Pipke, J., Boice, E., Pratt, L., Sherwood Lollar, B., Brodie, E., Hazen, T., Andersen, G., DeSantis, T., Moser, D., Kershaw, D., and Onstott, T.C., 2006, Long-term sustainability of a high-energy, low-diversity crustal biome: Science, v. 314, p. 479–482.
- Lowenstein, T.K., Li, J., Brown, C., Roberts, S.M., Ku, T.-L., Luo, S., and Yang, W., 1999, 200 k.y. paleoclimate record from Death Valley salt core: Geology, v. 27, p. 3–6.
- Lowenstein, T.K., Timofeeff, M.N., Brennan, S.T., Hardie, L.A., and Demicco, R.V., 2001, Oscillations in Phanerozoic seawater chemistry: Evidence from fluid inclusions: Science, v. 294, p. 1086–1088.
- McGenity, T.J., Gemmell, R.T., Grant, W.D., and Stan-Lotter, H., 2000, Origins of halophilic microorganisms in ancient salt deposits: Environmental Microbiology, v. 2, p. 243–250.
- Miteva, V.I., and Brenchley, J.E., 2005, Detection and isolation of ultrasmall microorganisms from a 120,000-year-old Greenland glacier ice core: Applied and Environmental Microbiology, v. 71, p. 7806–7818.
- Miteva, V.I., Sheridan, P.P., and Brenchley, J.E., 2004, Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core: Applied and Environmental Microbiology, v. 70, p. 202–213.
- Morita, R.Y., 1982, Starvation-survival of heterotrophs in the marine environment: Advances in Microbial Ecology, v. 6, p. 171–198.
- Morita, R.Y., 1997, Bacteria in Oligotrophic Environments: Starvation-Survival Lifestyle: New York, Chapman and Hall, 529 p.
- Mormile, M.R., Biesen, M.A., Gutierrez, M.C., Ventosa, A., Pavlovich, J.B., Onstott, T.C., and Fredrickson, J.K., 2003, Isolation of Halobacterium salinarum retrieved directly from halite brine inclusions: Environmental Microbiology, v. 5, p. 1094–1102.
- Norton, C.F., and Grant, W.D., 1988, Survival of halobacteria within fluid inclusions in salt crystals: Journal of General Microbiology, v. 134, p. 1365–1373.
- Novitsky, J.A., and Morita, R.Y., 1976, Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio: Applied and Environmental Microbiology, v. 32, p. 617–622.
- Onstott, T.C., Lin, L.-H., Davidson, M., Mislowack, B., Borcsik, M., Hall, J., Slater, G., Ward, J., Sherwood Lollar, B., Lippmann-Pipke, J., Boice, E., Pratt, L.-M., Pfiffner, S., Moser, D., Gihring, T., Kieft, T., Phelps, T., Vanheerden, E., Litthaur, D., Deflaun, M., Rothmel, R., Wanger, G., and Southam, G., 2006, The origin and age of biogeochemical trends in deep fracture water of the Witwatersrand Basin, South Africa: Geomicrobiology Journal, v. 23, p. 369–414.
- Oren, A., 2002a, Molecular ecology of extremely halophilic Archaea and Bacteria: FEMS Microbiology Ecology, v. 39, p. 1–7.
- Oren, A., 2002b, Halophilic microorganisms and their environments: Dordrecht, Netherlands, Kluwer Academic Publishers, 575 p.
- Oren, A., and Rodríguez-Valera, F., 2001, The contribution of halophilic Bacteria to the red coloration of saltern crystallizer ponds: FEMS Microbiology Ecology, v. 36, p. 123–130.
- Parkes, R.J., Cragg, B.A., and Wellsbury, P., 2000, Recent studies on bacterial populations and processes in subseafloor sediments: A review: Hydrogeology Journal, v. 8, p. 11–28.
- Pedrós-Alió, C., 2004, Trophic ecology of solar salterns, in Ventosa, A, ed., Halophilic Microorganisms: Berlin, Springer-Verlag, p. 33–48.
- Pedrós-Alió, C., Calderón-Paz, J.I., MacLean, M.H., Medina, G., Marrasé, C., Gasol, J.M., and Guixa-Boixereu, N., 2000, The microbial food web along salinity gradients: FEMS Microbiology Ecology, v. 32, p. 143–155.
- Radax, C., Gruber, C., and Stan-Lotter, H., 2001, Novel haloarchaeal 16S rRNA gene sequences from Alpine Permo-Triassic rock salt: Extremophiles, v. 5, p. 221–228.
- Reiser, R., and Tasch, P., 1960, Investigation of the viability of osmophile bacteria of great geological age: Transactions of the Kansas Academy of Science, v. 63, p. 31–34.
- Roedder, E., and Bassett, R.L., 1981, Problems in determination of the water content of rock-salt samples and its significance in nuclear-waste storage siting: Geology, v. 9, p. 525–530.
- Satterfield, C.L., Lowenstein, T.K., Vreeland, R.H., Rosenzweig, W.D., and Powers, D.W., 2005, New evidence for 250 Ma age of halotolerant bacterium from a Permian salt crystal: Geology, v. 33, p. 265–268.
- Schubert, B.A., Lowenstein, T.K., and Timofeeff, M.N., 2009a, Microscopic identification of prokaryotes in modern and ancient halite, Saline Valley and Death Valley, California: Astrobiology, v. 9, p. 467–482.
- Schubert, B.A., Lowenstein, T.K., Timofeeff, M.N., and Parker, M.A., 2009b, How do prokaryotes survive in fluid inclusions in halite for 30,000 years?: Geology, v. 37, p 1059–1062.
- Schubert, B.A., Lowenstein, T.K., Timofeeff, M.N., and Parker, M.A., 2010a, Halophilic Archaea cultured from ancient halite, Death Valley, California: Environmental Microbiology, v. 12, no. 2, p. 440–454.
- Schubert, B.A., Timofeeff, M.N., Polle, J.E., and Lowenstein, T.K., 2010b, Dunaliella cells in fluid inclusions in halite: Significance for long-term survival of prokaryotes: Geomicrobiology Journal, v. 27, no. 1, p. 61–75.
- Stan-Lotter, H., McGenity, T.J., Legat, A., Denner, E.B.M., Glaser, K., Stetter, K.O., and Wanner, G., 1999, Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits: Microbiology, v. 145, p. 3565–3574.
- Teller, J.T., 1987, The pink colour of lakes, with an example from Australia: Journal of Arid Environments, v. 12, p. 101–103.
- Vreeland, R.H., Rosenzweig, W.D., and Powers, D.W., 2000, Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal: Nature, v. 407, p. 897–900.
- Vreeland, R.H., Jones, J., Monson, A., Rosenzweig, W.D., Lowenstein, T.K., Timofeeff, M., Satterfield, C., Cho, B.C., Park, J.S., Wallace, A., and Grant, W.D., 2007, Isolation of live Cretaceous (121–112 million years old) halophilic Archaea from primary salt crystals: Geomicrobiology Journal, v. 24, p. 275–282.
- Willerslev, E., Hansen, A.J., and Poinar, H.N., 2004, Isolation of nucleic acids and cultures from fossil ice and permafrost: Trends in Ecology & Evolution, v. 19, p. 141–147.
- Willerslev, E., and Hebsgaard, M.B., 2005, New evidence for 250 Ma age of halotolerant bacterium from a Permian salt crystal: Comment: Geology, p. e93, doi: 10.1130/0091-7613-33.1.e93.

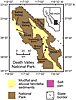 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3 Figure 4
Figure 4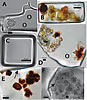 Figure 5
Figure 5