Full Text View
Volume 19 Issue 11 (November 2009)
GSA Today
Article, pp. 4-10 | Abstract | PDF (829KB)
Geobiology: Evidence for early life on Earth and the search for life on other planets
| Table of Contents |
|---|
| Search GoogleScholar for
Search GSA Today |
Extensive research efforts in the subdisciplinary field of geobiology have focused on the interactions between Earth and life through time. As a consequence, gaps in our knowledge of Earth’s history are closing, and the search for life beyond Earth is expanding. A few examples of geobiology studies designed to advance our understanding of life on early Earth and to improve the chances of finding life on other planets are provided to highlight recent developments and research areas that are on the verge of new discoveries.
Manuscript received 22 June 2009; accepted 5 August 2009.
doi: 10.1130/GSATG62A.1
*E-mails: ,
INTRODUCTION
A central theme in geobiology is the coevolution of biological and surficial geological processes. As illustrated in Figure 1, the synthesis of data sets gleaned from modern ecosystems, ancient deposits, and experimental systems enables geobiologists to test hypotheses generated from key interdisciplinary questions. Such an integrated approach makes it possible to refine increasingly sophisticated models designed to reconstruct past environmental and evolutionary events, predict future fluctuations over a range of spatial and temporal scales, and improve experimental study of the influence of biology on chemical and physical processes, and vice versa. Never before has the potential for interdisciplinary research among geoscientists and bioscientists been more fruitful, as is reflected in our expanding comprehension of Earth’s history and early life.
|
Schematic flow chart illustrating the logical outgrowth of the interdisciplinary science of geobiology. As hypotheses that involve topics and concepts from more than one discipline (i.e., from the parent disciplines geology and biology and related subdisciplines) arise, interdisciplinary approaches can be applied to study ancient deposits, experimental (abiotic and biotic) systems, and modern ecosystems. When such data sets are synthesized with one another in an iterative fashion, a new level of understanding can be achieved, one that makes it possible to reconstruct parts of Earth’s history, expand our range of understanding across temporal (e.g., since the Archean Eon) and spatial (e.g., greater than planetary) scales, and refine our experimental approaches in laboratory and field settings. Results that improve the ability to model future events across the full range of spatial and temporal scales are particularly important at this time in Earth’s history and allow us to predict the future impact of life on our planet. All of these outcomes can feed back into concepts needed to advance the parent disciplines of geology and biology and the subdisciplines of geobiology. They also typically contribute to fields like astrobiology. Illustration modified after Noffke (2005). |
LIFE’S IMPRINT—DECIPHERING ANCIENT BIOSIGNATURES
An understanding of the coevolution of life and its physical and chemical settings relies on the ability to decipher evidence of life preserved in the rock record. While any phenomenon produced by life (modern or ancient) can be considered a biosignature (cf. Steele et al., 2005), the main challenge in ancient and extraterrestrial life detection is determining whether the phenomenon (or suite of phenomena) can be uniquely attributed to life. Taphonomic changes inevitably alter the chemical and structural fidelity of all biosignatures over time.
Biosignatures of microorganisms fall into one of three categories (Cady et al., 2003): (1) bona fide cellular fossils (cf. Cady, 2002) and carbonaceous remnants of microbial cells and their extracellular matrices (Cady, 2001); (2) microbially influenced fabrics and sedimentary structures (which include some laminated stromatolites; cf. Grotzinger and Knoll, 1999); and (3) chemical fossils (e.g., organic compounds, such as biomarkers; inorganic phases, such as some minerals, mineraloids, and gases; stable isotopic patterns associated with life in organic and inorganic constituents; and disequilibrium phase enrichments [Des Marais et al., 2008a]).
A dramatic secular change in Earth’s history has been the impact of life on the diversity of minerals. Hazen et al. (2008) estimated that, over the past 4.56 billion years, the number of different minerals has increased from about a dozen to more than 4300 known types. Though only a small number of these can be considered biominerals (i.e., chemical fossils), even their use as definitive evidence for life remains, justifiably, problematic (e.g., Golden et al., 2004; Altermann et al., 2009). In any case, biology has altered the relative abundances of different groups of minerals (most notably since the oxidation of the atmosphere), expanded the range of compositional variants (which include solid solutions and minor and trace element variations), affected the kinetics of mineral formation (hence the degree of ordering and density/type of defect microstructure), and created distinctive morphological habits. The emergence of key microbial metabolic innovations throughout Earth’s history and development of bioskeletons during the Phanerozoic resulted in the biomineralization mechanisms that persist today (cf. Ehrlich and Newman, 2009). Collectively, the diverse metabolic and behavioral activities of life have created and sustained chemical gradients in geochemically dynamic environments, which has led to an abundance of mineral varieties distributed over scales that range from microenvironments around, and within, cells to regional-sized terrains.
Deciphering biosignatures and evidence of microbial activity in ancient rock remains a central challenge in geobiology studies (e.g., Rosing, 1999; Fedo and Whitehouse, 2002; Lepland et al., 2005). When surface-derived, organic-bearing rocks are transferred to Earth’s shallow interior, the combination of burial and deformation can ultimately make it impossible to distinguish a biological signature in relict carbonaceous compounds (Pasteris and Wopenka, 2003; Brasier et al., 2005). Consequently, determination of the degree of metamorphism beyond which life’s signatures are no longer recognizable in ancient carbon is a research topic of considerable interest in early Earth and extraterrestrial studies (e.g., Schopf and Kudryavtsev, 2009; Glikson et al., 2008; Oehler et al., 2009). For example, a recent approach focuses on the applicability and limitations of using Raman spectroscopy to characterize evidence of ancient life (e.g., van Zuilen et al., 2002; Schopf et al., 2005; McKeegan et al., 2007; Schiffbauer et al., 2007; van Zuilen et al., 2007; Marshall et al., 2007).
Isotopic studies continue to reveal new insight about the range of metabolic diversity on early Earth. For example, isotopic evidence for ancient sulfur-based metabolisms has recently been advanced by the use of integrated 32S, 33S, and 34S isotopic studies of sulfides and sulfates from chert-barite deposits at North Pole, northwestern Australia (Dresser Formation). Though the record of heavy sulfur (34S/33S) isotopes in microscopic sulfides preserved in early Archean barites has been known for some time (Shen et al., 2001), recent analyses of North Pole samples indicate that the combination of negative δ34S and positive δ33S values of these sulfides cannot be accounted for by microbial sulfate reduction (Philippot et al., 2007, 2008; Ueno et al., 2008). Microbial disproportionation of elemental sulfur is proposed as an alternative to sulfate reduction to explain the anomalous isotopic character of Archean sulfides from the Dresser Formation (Philippot et al., 2007, 2008).
New evidence for a wider range of diversity in early metazoans and the microbial communities with which they lived has been found in recent years (e.g., Narbonne, 2005), in part because of the application of new analytical tools to characterize the morphology of these ancient life forms. A particularly rich contribution to our understanding of early metazoan life has come from synchrotron-radiation X-ray tomographic microscopy studies of the lower Ediacaran Doushantuo Formation in the Yangtze Gorges area in China, which contains centimeter-sized chert nodules that preserve metazoans, cyanobacteria, multicellular algae, spiny acritarchs, and animal eggs and embryos (Hagadorn et al., 2006; Donoghue et al., 2006; Xiao et al., 2007). For a recent review of the variety of synchrotron-based X-ray spectroscopy and microscopy techniques, see Templeton and Knowles (2009). It is worth noting that the application of a variety of nanotomographic techniques is on the rise. For example, three-dimensional renderings of different forms of acritarchs have been obtained with the use of optical microscope (Sugitani et al., 2009) and focused ion-beam (Kempe et al., 2005; Schiffbauer and Xiao, 2009) nanotomography.
MODERN ECOSYSTEM AND EXPERIMENTAL STUDIES: INSIGHTS INTO EARLY LIFE ACTIVITIES
Though the use of modern analog (similar but not identical) settings to gain insight into the processes that may have occurred in ancient environments is not new, such strategies have been key drivers in recent geobiology studies. For example, an ancient sedimentary deposit inextricably linked to biological activity is the banded iron formation (BIF). These iron-rich (~20%–40% Fe) siliceous (~40%–50% SiO2) rocks, which often contain carbonate and sulfide facies, accumulated as sediments throughout much of the late Archean (2.7–2.5 Ga) and Paleoproterozoic (2.5–1.8 Ga) (e.g., Trendall, 2002; Klein, 2005). Though the mineralogy of BIFs dictates that some oxidation of Fe(II) had to have occurred, the relative contributions and nature of different types of abiotic and biotic (oxygenic photosynthesizers and Fe2+ oxidizers) mechanisms responsible for the formation of the iron in these deposits continues to be debated. Recent studies of ancient BIFs indicate that bacteria could have contributed in a number of ways to the accumulation of these visually stunning ancient deposits, which formed as sedimentary precipitates (e.g., Konhauser et al., 2002, 2007; Kappler et al., 2005; Johnson et al., 2008; Planavsky et al., 2009). Efforts to elucidate BIF accumulation mechan-isms by studying modern analog ecosystems (e.g., Trouwborst et al., 2007; Parenteau and Cady, 2009) support the early hypothesis of Cloud (1965), which stressed the key role played by cyanobacteria. Reconciliation of theoretical arguments that focus on the range of possible microbial impacts with results from studies of modern ecosystems and ancient deposits may be possible by way of another approach essential to geobiology (e.g., Fig. 1); that is, a methodology based on the inclusion of experimental studies.
An example of an experimental approach carried out in a modern ecosystem involves recent studies of microbially induced sedimentary structures (MISS) (Noffke and Paterson, 2008). Given that physical interactions between microbes and their environment are unlikely to have changed in a significant way throughout Earth’s history, actualistic studies of such interactions can reveal the various ways in which microbial life affects the accumulation of detrital sediments. Studies in modern settings make it possible to observe and quantify the response of benthic microbiota to physical sediment dynamics. Biostabilization (Fig. 2) and baffling, trapping, and binding of microbiota associated with loose sediments generate a multitude of MISS (Noffke, 2009). For example, the sediment-stabilizing properties of the indigenous microbial consortium can be measured with a portable Manzenrieder flume chamber deployed in a modern ecosystem (Fig. 2A). In this experiment, an artificial water current that crosses the microbial mat surface is produced. A digital system analyzes the first release of sand grains from the flume chamber, an event that marks the start of erosion of the microbial mat. The effect of the microbial consortium on biostabilization of the sandy deposits is illustrated by the Shield’s diagram in Figure 2B. Endobenthic microbial mats that colonize the uppermost millimeter of the sandy tidal surface reduce the erosive forces of the currents by 3–5 times compared to sterile sand (stars, Fig. 2B). Therefore, the mat-covered sand withstands currents of up to 0.90 cm/s. The biostabilization effect is caused by the lower degree of roughness of the mat-interwoven sedimentary surface. Since the grains do not protrude through the viscous sublayer, the flow across a microbial mat is hydrodynamically smooth, and only laminar flow, not the more intensive turbulent stress, affects the mat surface. Epibenthic microbial mats that cover the tidal sands like a carpet reduce the erosive forces up to magnitudes of 12 (dots, Fig. 2B). As a consequence, such thick mats withstand currents of up to 1.60 m/s. This biostabilization effect is due to the “slippery” mat surface, which prevents the direct influence of turbulent waters on the sand grains. This microbial effect can be expressed by a simple modification of the Shield’s relation for sediment movement:
![]() (1)
(1)
where u* is the shear velocity; ρf is the density of fluid; ρs is the density of sediment; g is the gravity constant; D is the actual grain diameter under the influence of biostabilization; and n is the exponent to which D is raised for the data to comply to the Shield’s relationship (cf. Führböter and Manzenrieder, 1987). Microbial sediment fixation is well documented in field and laboratory experiments (e.g., Neumann et al., 1970; De Boer, 1981; Grant, 1988, Dade et al., 1990; Schieber, 2007; and contributions in Noffke and Paterson, 2008).
|
An example of a geobiological approach to the study of biostabilization of sediment by a microbial consortium (e.g., a microbial mat or biofilm). Photograph: Portable Manzenrieder flume chamber on a modern tidal flat surface. The Shield’s diagram illustrates the impact of biostabilizing microbial mats on the potential for sediment transport. Dots—values of biostabilization by epibenthic microbial mats; stars—values of biostabilization by endobenthic microbial mats. Measurements from Portsmouth Island, USA, Aug. 2003–Nov. 2006, following Führböter and Manzenrieder, 1987. |
RECOGNIZING BIOSIGNATURES IN EXTRATERRESTRIAL SYSTEMS
Geobiological approaches provide a foundation for astrobiological studies that focus on the search for extraterrestrial life on other planetary bodies. Conceptual frameworks for research in astrobiology (Des Marais et al., 2008b; Worms et al., 2009) pose the most intriguing questions in this field of inquiry: How does life begin and evolve? Does life exist elsewhere? What is the future of life on Earth and beyond? The possibility that Mars samples could be returned to Earth in our lifetime provides additional impetus to identify and characterize a wide range of biosignatures, even if they are present in minute amounts and altered from their pristine state (Farmer et al., 2009). Geobiology studies of ancient environmental settings, where life could have thrived, or of modern ecosystems, especially extreme ecosystems, are key in this regard. It has become apparent that a wide variety of environmental settings (e.g., Nisbet and Sleep, 2001) may have supported a diverse range of anaerobic (Canfield et al., 2006) and extremophilic life on early Earth (e.g., Rothschild and Mancinelli, 2001).
It is possible that life emerged and became widespread on Earth prior to the Archean. Abramov and Mojzsis (2009) have used thermal models to argue that life would have persisted in subsurface niches during the late heavy bombardment period, a time when Earth’s surface was being reworked by impactors of all sizes. Such findings reinvigorate the hypothesis that widespread hydrothermal activity, which produced subsurface biomes for chemotrophic hyperthermophilic communities, facilitated life’s emergence and early diversification (Pace, 1997). Carbonaceous morphological remains of subsurface biofilms have now been found in hydrothermal precipitates produced by meteorite impacts (Hode et al., 2008, Fig. 3). Given the variety of fossil biosignatures likely to survive in hydrothermal deposits (e.g., Reysenbach and Cady, 2001; Konhauser et al., 2003), the possibility that ancient microbial life survived in hydrothermal niches has important implications for those involved in the search for ancient and extraterrestrial life (e.g., Farmer and Des Marais, 1999). Rock outcrops that could have resulted from hydrothermal activity on Mars have recently been reported (Squyres et al., 2008; Allen and Oehler, 2008).
|
Examples of possible biosignatures revealed after chemical etching of calcite-filled veins that formed as a result of impact-induced hydrothermal activity associated with the Siljan Impact Structure, Sweden (see Hode et al., 2008, and references therein, for information on sample preparation and analytical methods). (A) Scanning electron microscope (SEM) photomicrograph montage of three low-magnification images provides an overview of the locations shown in B–G (arrows). Pyrite assemblages comprise the topographical highs because etching removed the top few tenths of micrometers of the surrounding calcite. Crack along the right side of image is the center of the hydrothermal vein. (B) Bundle of thread-shaped features shown in center of SEM image. (C) Adjacent area includes curved and torn features still partly embedded in the calcite crystal. (D) Filamentous feature attached to and extended between pyrite (topographical high) and the calcite matrix. (E) This perforated carbonaceous film (arrow) between a pyrite crystal and the calcite matrix was exposed after etching. A nuclear microprobe was used to identify the carbonaceous composition of the biofilm remnant. (F) Film-like feature wrapped around the edge of a pyrite aggregate (arrow) is fully pyritized as no evidence for carbonaceous matter could be found. Etching has removed the surrounding calcite and left the pyrite exposed as topographical highs. (G) Pyrite framboid inside the calcite matrix. Pyrite framboids are often found in reducing hydrothermal systems rich in carbonaceous matter. Figure originally published in Hode et al. (2008) and reprinted with permission. |
Stromatolites have remained essential biological mileposts throughout Earth’s history and are associated with a diverse range of microbial communities and environments (e.g., Reid et al., 2000; Grotzinger and Knoll, 1999; Cady et al., 2003; Allwood et al., 2007). Paleoarchean stromatolites, in particular, reveal the nature of Earth’s earliest biosphere and the environmental conditions that supported and led to the preservation of this evidence for early life (e.g., Allwood et al., 2009). Studies of modern and equivalent fossil microbially induced sedimentary structures, the sandy counterpart of stromatolites (Noffke, 2009), will likewise be helpful in recognizing Earth’s oldest environments and deciphering life’s imprint on such structures, should they be found on Mars.
CONCLUSION
The topics covered here exemplify some of the most recent approaches in geobiology and illustrate the link with astrobiology. They also serve to remind us that, though some of the most intriguing questions about early life’s impact on rocky planets are yet to be answered, a geobiological approach is essential to our understanding of life and the role it has played throughout Earth’s history.
REFERENCES CITED
- Abramov, O., and Mojzsis, S.J., 2009, Microbial habitability of the Hadean Earth during the late heavy bombardment: Nature, v. 459, p. 419-422, doi: 10.1038/nature08015.
- Allen, C.C., and Oehler, D.Z., 2008, A case for ancient springs in Arabia Terra: Mars: Astrobiology, v. 8, no. 6, p. 1093-1112, doi: 10.1089/ast.2008.0239.
- Allwood, A.C., Walter, M.R., Burch, I.W., and Kamber, B.S., 2007, 3.43 billion-year-old stromatolite reef from the Pilbara Craton of Western Australia: Ecosystem-scale insights to early life on Earth: Precambrian Research, v. 158, p. 198-227, doi: 10.1016/j.precamres.2007.04.013.
- Allwood, A.C., Grotzinger, J.P., Knoll, A.H., Burch, I.W., Anderson, M.S., Coleman, M.L., and Kanika, I., 2009, Controls on development and diversity of early Archean stromatolites: Proceedings of the National Academy of Sciences of the United States of America, v. 106, no. 24, p. 9548-9555, doi: 10.1073/pnas.0903323106.
- Altermann, W., Böhmer, C., Gitter, F., Heimann, F., Heller, I., Läuchli, B., and Putz, C., 2009, Discussion about “Defining biominerals and organominerals”: Sedimentary Geology, v. 213, p. 150-151, doi: 10.1016/j.sedgeo.2008.04.001.
- Brasier, M.D., Green, O.R., Lindsay, J.F., McLoughlin, N., Steele, A., and Stoakes, C., 2005, Critical testing of Earth’s oldest putative fossil assemblage from the ~3.5 Ga Apex chert, Chinaman Creek, Western Australia: Precambrian Research, v. 140, p. 55-102, doi: 10.1016/j.precamres.2005.06.008.
- Cady, S.L., 2001, Paleobiology of the Archean, in Blum, P., ed., Archaea: Ancient Microbes, Extreme Environments, and the Origin of Life: Advances in Applied Microbiology, v. 50, p. 1-35.
- Cady, S.L., 2002, Formation and preservation of bona fide microfossils, in Signs of Life: A report based on the April 2000 Workshop on Life-Detection Techniques: The National Academies Space Studies Board and Board on Life Sciences, Carnegie Institution of Washington, National Academies Press, Washington, D.C., p. 149-155.
- Cady, S.L., Farmer, J.D., Grotzinger, J.P., Schopf, J.W., and Steele, A., 2003, Morphological biosignatures and the search for life on Mars: Astrobiology, v. 3, no. 2, p. 351-368, doi: 10.1089/153110703769016442.
- Canfield, D.E., Rosing, M.T., and Bjerrum, C., 2006, Early anaerobic metabolisms: Philosophical Transactions of the Royal Society B: Biological Science, v. 361, p. 1819-1836, doi: 10.1098/rstb.2006.1906.
- Cloud, P.E., 1965, Significance of the Gunflint (Precambrian) microflora: Science, v. 148, p. 27-35, doi: 10.1126/science.148.3666.27.
- Dade, W.B., Davis, J.D., Nichols, P.D., Nowell, A.M., Thistle, D., Trexler, M.B., and White, D.C., 1990, Effects of bacterial exopolymer adhesion on the entrainment of sand: Geomicrobiology Journal, v. 8, p. 1-16, doi: 10.1080/01490459009377874.
- De Boer, P.L., 1981, Mechanical effects of microorganisms on intertidal bedform migration: Sedimentology, v. 28, p. 129-132, doi: 10.1111/j.1365-3091.1981.tb01670.x.
- Des Marais, D.J., and Jakosky, B.M. and Hynek, B.M., 2008a, Astrobiological Implications for Mar’s Surface Composition and Properties, in Bell, J.F., III, ed., The Martian Surface: Composition, Mineralogy, and Physical Properties: Cambridge University Press, p. 599-623.
- Des Marais, D.J., Nuth, J.A., III, Allamandola, L.J., Boss, A.P., Farmer, J.D., Hoehler, T.M., Jakosky, B.M., Meadows, V.S., Pohorille, A., Runnegar, B., and Spormann, A.M., 2008b, The NASA Astrobiology Roadmap: Astrobiology, v. 8, no. 4, p. 715-730, doi: 10.1089/ast.2008.0819.
- Donoghue, P.C.J., Bengtson, S., Dong, X.-P., Gostling, N.J., Huldtgren, T., Cunningham, J.A., Yin, C., Yue, Z., Peng, F., and Stampanoni, M., 2006, Synchrotron X-ray tomographic microscopy of fossil embryos: Nature, v. 442, p. 680-683, doi: 10.1038/nature04890.
- Ehrlich, H.L., and Newman, D.K., 2009, Geomicrobiology, Fifth Edition: Taylor and Francis Group, CRC Press, 606 p.
- Farmer, J.D., and Des Marais, D.J., 1999, Exploring for a record of ancient martian life: Journal of Geophysical Research, Planets, v. 104, E11, p. 26,977-26,995, doi: 10.1029/1998JE000540.
- Farmer, J.D., Bell, J.F., III, Benison, K.C., Boynton, W.V., Cady, S.L., Ferris, F.G., MacPherson, D., Race, M.S., Thiemens, M.H., and Wadhwa, M., 2009, Assessment of Planetary Protection Requirements for Mars Sample Return Missions: Washington, D.C., The National Academies Press, 80 p.
- Fedo, C.M., and Whitehouse, M.J., 2002, Metasomatic origin of quartz-pyroxene rock, Akilia, Greenland, and implications for Earth’s earliest life: Science, v. 296, p. 1448-1452, doi: 10.1126/science.1070336.
- Führböter, A., and Manzenrieder, H., 1987, Biostabilisierung von Sandwatten durch Mikrooranismen, in Gerdes, G., Krumbein, W.E., and Reineck, H.E., eds., Mellum—Porträit einer Insel: Franfurt am Main, Kramer, p. 123-138.
- Glikson, M., Duck, L.J., Golding, S.D., Hofmann, A., Bolhar, R., Webb, R., Baiano, J.C.F., and Sly, L.I., 2008, Microbial remains in some earliest Earth rocks: Comparison with a potential modern analogue: Precambrian Research, v. 164, no. 3-4, p. 187-200, doi: 10.1016/j.precamres.2008.05.002.
- Golden, D.C., Ming, D.W., Morris, R.V., Brearley, A.J., Lauer, H.V., Jr., Treiman, A.H., Zolensky, M.E., Schwandt, C.S., Lofgren, G.E., and McKay, G.A., 2004, Evidence for exclusively inorganic formation of magnetite in martian meteorite ALH84001: The American Mineralogist, v. 89, p. 681-695.
- Grant, J., 1988, Intertidal bedforms, sediment transport, and stabilization by benthic microalgae, in de Boer, P.L., van Gelder, A., and Nio, S.D., eds., Tide-influenced sedimentary environments and facies: Dordrecht, Reidel, p. 499-510.
- Grotzinger, J.P., and Knoll, A.H., 1999, Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks?: Annual Review of Earth and Planetary Sciences, v. 27, p. 313-358, doi: 10.1146/annurev.earth.27.1.313.
- Hagadorn, J.W., Xiao, S., Donoghue, P.C.J., Bengtson, S., Gostling, N.J., Pawlowska, M., Raff, E.C., Raff, R.A., Turner, F.R., Yin, C., Zhou, C., Yuan, X., McFeely, M.B., Stampanoni, M., and Nealson, K.H., 2006, Cellular and subcellular structure of Neoproterozoic embryos: Science, v. 314, p. 291-294, doi: 10.1126/science.1133129.
- Hazen, R.M., Papineau, D., Bleeker, W., Downs, R.T., Ferry, J.M., McCoy, T.J., Sverjensky, D.A., and Yang, H., 2008, Mineral evolution: The American Mineralogist, v. 93, p. 1693-1720, doi: 10.2138/am.2008.2955.
- Hode, T., Cady, S.L., von Dalwigk, I., and Kristiansson, P., 2008, Evidence of ancient microbial life in a large impact structure and its implications for astrobiology: A case study, in Seckbach, J., and Walsh, M., eds., From Fossils to Astrobiology: Berlin, Springer Science Series Volume 12—Cellular Origin, Life in Extreme Habitats and Astrobiology, p. 249-273.
- Johnson, C.M., Beard, B.L., Klein, C., Beukes, N.J., and Roden, E.E., 2008, Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis: Geochimica et Cosmochimica Acta, v. 72, p. 151-169, doi: 10.1016/j.gca.2007.10.013.
- Kappler, A., Pasquero, C., Konhauser, K.O., and Newman, D.K., 2005, Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria: Geology, v. 33, p. 865-868, doi: 10.1130/G21658.1.
- Kempe, A., Wirth, R., Wladyslaw, A., Stark, R.W., Schopf, J.W., and Heckl, W.M., 2005, Focussed ion beam preparation and in situ nanoscopic study of Precambrian acritarchs: Precambrian Research, v. 140, p. 36-54, doi: 10.1016/j.precamres.2005.07.002.
- Klein, C., 2005, Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins: The American Mineralogist, v. 90, no. 10, p. 1473-1499, doi: 10.2138/am.2005.1871.
- Konhauser, K.O., Hamade, T., Raiswell, R., Morris, R.C., Ferris, F.G., Southam, G., and Canfield, D.E., 2002, Could bacteria have formed the Precambrian banded iron formations?: Geology, v. 30, p. 1079-1082, doi: 10.1130/0091-7613(2002)030<1079:CBHFTP>2.0.CO;2.
- Konhauser, K.O., Jones, B., Reysenbach, A.-L., and Renaut, R.W., 2003, Hot spring sinters: keys to understanding Earth’s earliest life forms: Canadian Journal of Earth Sciences, v. 40, no. 11, p. 1713-1724, doi: 10.1139/e03-059.
- Konhauser, K.O., Amskold, L., Lalonde, S.V., Posth, N.R., Kappler, A., and Anbar, A., 2007, Decoupling photochemical Fe(II) oxidation from shallow-water deposition: Earth and Planetary Science Letters, v. 258, p. 87-100, doi: 10.1016/j.epsl.2007.03.026.
- Lepland, A., van Zuilen, M.A., Arrhenius, G., Whitehouse, M.J., and Fedo, C.M., 2005, Questioning the evidence for Earth’s earliest life—Akilia revisited: Geology, v. 33, p. 77-79, doi: 10.1130/G20890.1.
- Marshall, C.P., Love, G.D., Snape, C.E., Hill, A.C., Allwood, A.C., Walter, M.R., Van Kranendonk, M.J., Bowden, S.A., Sylva, S.P., and Summons, R.E., 2007, Structural characterization of kerogen in 3.4 Ga Archaean cherts from the Pilbara Craton, Western Australia: Precambrian Research, v. 155, p. 1-23, doi: 10.1016/j.precamres.2006.12.014.
- McKeegan, K.D., Kudryantsev, A.B., and Schopf, J.W., 2007, Raman and ion microscopic imagery of graphitic inclusions in apatite from older than 3830 Ma Akilia supracrustal rocks, west Greenland: Geology, v. 35, p. 591-594, doi: 10.1130/G23465A.1.
- Narbonne, G.M., 2005, The Ediacara biota: Neoproterozoic origin of animals and their ecosystems: Annual Review of Earth and Planetary Sciences, v. 33, p. 421-442, doi: 10.1146/annurev.earth.33.092203.122519.
- Neumann, A.C., Gebelein, C.D., and Scoffin, T.P., 1970, The composition, structure, and erodability of subtidal mats, Abaco, Bahamas: Journal of Sedimentary Petrology, v. 40, p. 247-297.
- Nisbet, E.G., and Sleep, N.H., 2001, The habitat and nature of early life: Nature, v. 409, p. 1083-1091, doi: 10.1038/35059210.
- Noffke, N., 2005, Geobiology—A holistic scientific discipline, in Noffke, N., ed., Geobiology: Objectives, Concepts, Perspectives: Paleoclimatology, Paleooceanography, Paleoecology (Special Issue): Amsterdam, Elsevier, p. 1-2.
- Noffke, N., 2009, The criteria for the biogenicity of microbially induced sedimentary structures (MISS) in Archean and younger, sandy deposits: Earth-Science Reviews, doi: 10.1016/j.earscirev.2008.08.002 (in press).
- Noffke, N., and Paterson, D., 2008, An Microbial interactions with physical sediment dynamics, and their significance for the interpretation of Earth’s biological history: Geobiology, v. 6 no.1, Special Issue.
- Oehler, D.Z., Robert, F., Walter, M.R., Sugitani, K., Allwood, A., Meibom, A., Mostefaoui, S., Selo, M., Thomen, A., and Gibson, E.K., 2009, NanoSIMS: Insights to biogenicity and syngeneity of Archaean carbonaceous structures: Precambrian Research, doi: 10.1016/j.precamres.2009.01.001 (in press).
- Pace, N.R., 1997, A molecular view of microbial diversity and the biosphere: Science, v. 276, p. 734-740, doi: 10.1126/science.276.5313.734.
- Parenteau, M.N., and Cady, S.L., 2009, Microbial biosignatures in iron-mineralized phototrophic mats at Chocolate Pots hot springs, Yellowstone National Park, USA: Palaios (in press). Pasteris, J.D., and Wopenka, B., 2003, Necessary, but not sufficient: Raman identification of disordered carbon as a signature of ancient life: Astrobiology, v. 3, no. 4, p. 727-738, doi: 10.1089/153110703322736051.
- Philippot, P., van Zuilen, M.A., Lepot, K., Thomazo, C., Farquhar, J., and Van Kranendonk, M.J., 2007, Early Archaean microorganisms preferred elemental sulfur, not sulfate: Science, v. 317, p. 1534-1537, doi: 10.1126/science.1145861.
- Philippot, P., Van Zuilen, M.A., Lepot, K., Thomazo, C., Farquhar, J., and Van Kranendonk, M.J., 2008, Response to Comment on “Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate”: Science, v. 319, p. 1336, doi: 10.1126/science.1151414.
- Planavsky, N., Rouxel, O., Bekker, A., Shapiro, R., Fralick, P., and Knudsen, A., 2009, Iron-oxidizing microbial ecosystems thrived in late Paleoproterozoic redox-stratified oceans: Earth and Planetary Science Letters, doi: 10.1016/j.epsl.2009.06.033 (in press).
- Reid, R.P., Visscher, P.T., Decho, A.W., Stolz, J.F., Bebout, B.M., Dupraz, C., MacIntyre, I.G., Pearl, H.W., Pinckney, J.L., Prufert-Bebout, L., Steppe, T.F., and Des Marais, D.J., 2000, The role of microbes in accretion, lamination and early lithification of modern marine stromatolites: Nature, v. 406, p. 989-991, doi: 10.1038/35023158.
- Reysenbach, A.-L., and Cady, S.L., 2001, Microbiology of ancient and modern hydrothermal systems: Trends in Microbiology, v. 9, p. 79-86, doi: 10.1016/S0966-842X(00)01921-1.
- Rosing, M.T., 1999, 13-C depleted carbon microparticles in >3700 Ma seafloor sedimentary rocks from West Greenland: Science, v. 283, p. 674-676, doi: 10.1126/science.283.5402.674.
- Rothschild, L.J., and Mancinelli, R.L., 2001, Life in extreme environments: Nature, v. 409, p. 1092-1101, doi: 10.1038/35059215.
- Schieber, J., 2007, Flume experiments on the durability of sandy microbial mat fragments during transport, in Schieber, J., Bose, P.K., Eriksson, P.G., Banerfjee, S., Sarkar, S., Altermanm, W., and Catuneau, O., eds., Atlas of microbial mat features preserved within the siliciclastic rock record: Amsterdam, Elsevier, p. 248-257.
- Schiffbauer, J.D., and Xiao, S., 2009, Novel application of focused ion beam electron microscopy (FIB-EM) in preparation and analysis of microfossil ultrastructures: A new view of complexity in early eukaryotic organisms: Palaios, v. 24, p. 616-626, doi: 10.2110/palo.2009.p09-003r.
- Schiffbauer, J.D., Yin, L., Bodnar, R.J., Kaufman, A.J., Meng, F., Hu, J., Shen, B., Yuan, X., Bao, H., and Xiao, S., 2007, Ultrastructural and geochemical characterization of Archean-Paleoproterozoic graphite particles: Implications for recognizing traces of life in highly metamorphosed rocks: Astrobiology, v. 7, p. 684-704, doi: 10.1089/ast.2006.0098.
- Schopf, J.W., and Kudryavtsev, A.B., 2009, Confocal laser scanning microscopy and Raman imagery of ancient microscopic fossils: Precambrian Research, doi: 10.1016/j.precamres.2009.02.007 (in press).
- Schopf, J.W., Kudryavtsev, A.B., Agresti, D.G., Czaja, A.D., and Wdowiak, T.J., 2005, Raman imagery: A new approach to assess the geochemical maturity and biogenicity of permineralized Precambrian fossils: Astrobiology, v. 5, p. 333-371, doi: 10.1089/ast.2005.5.333.
- Shen, Y., Buick, R., and Canfield, D.E., 2001, Isotopic evidence for microbial sulphate reduction in the early Archean era: Nature, v. 410, p. 77-81, doi: 10.1038/35065071.
- Squyres, S.W., Arvidson, R.E., Ruff, S., Gellert, R., Morris, R.V., Ming, D.W., Crumpler, L., Farmer, J.D., Des Marais, D.J., Yen, A., McLennan, S.M., Calvin, W., Bell, J.F., III, Clark, B.C., Wang, A., McCoy, T.J., Schmidt, M.E., and de Souza, P.A., Jr., 2008, Detection of silica-rich deposits on Mars: Science, v. 320, p. 1063-1067, doi: 10.1126/science.1155429.
- Steele, A., Beaty, D.W., Amend, J., Anderson, R., Beegle, L., Benning, L., Bhattacharya, J., Blake, D., Brinckerhoff, W., Biddle, J., Cady, S., Conrad, P., Lindsay, J., Mancinelli, R., Mungas, G., Mustard, J., Oxnevad, K., Toporski, J., and Waite, H., 2005, The Astrobiology Field Laboratory: JPL Document Review Services (Reference #CL#06-3307), Mars Exploration Program Analysis Group (MEPAG), Unpublished white paper, 72 p., http://mepag.jpl.nasa.gov/reports/index.html [posted Dec. 2005; last accessed Aug. 2009].
- Sugitani, K., Grey, K., Nagaoka, T., and Mimura, K., 2009, Three-dimensional morphological and textural complexity of Archean putative microfossils from the northeastern Pilbara Craton: Indications of biogenicity of large (>15μm) spheroidal and spindle-like structures: Astrobiology, v. 9 (in press).
- Templeton, A., and Knowles, E., 2009, Microbial transformations of minerals and metals: Recent advances in geomicrobiology derived from synchrotron-based X-ray spectroscopy and X-ray microscopy: Annual Review of Earth and Planetary Sciences, v. 37, p. 367-391, doi: 10.1146/annurev.earth.36.031207.124346.
- Trendall, A.L., 2002, The significance of iron-formation in the Precambrian stratigraphic record, in Altermann, W., and Corcoran, P.L., eds., Precambrian Sedimentary Environments: A Modern Approach to Ancient Depositional Systems: International Association of Sedimentologists (IAS) Special Publication 33, Blackwell Science, p. 33-66.
- Trouwborst, R.E., Johnston, A., Koch, G., and Pierson, B.K., 2007, Biogeochemistry of Fe(II) oxidation in a photosynthetic microbial mat: Implications for Precambrian Fe(II) oxidation: Geochimica et Cosmochimica Acta, v. 71, p. 4629-4643, doi: 10.1016/j.gca.2007.07.018.
- Ueno, Y., Ono, S., Rumble, D., and Maruyama, S., 2008, Quadruple sulfur isotope analysis of ca. 3.5 Ga Dresser Formation: New evidence for microbial sulfate reduction in the early Archean: Geochimica et Cosmochimica Acta, v. 72, p. 5675-5691, doi: 10.1016/j.gca.2008.08.026.
- van Zuilen, M.A., Lepland, A., and Arrhenius, G., 2002, Reassessing the evidence for the earliest traces of life: Nature, v. 418, p. 627-630, doi: 10.1038/nature00934.
- van Zuilen, M.A., Chaussidon, M., Rollion-Bard, C., and Marty, B., 2007, Carbonaceous cherts of the Barberton Greenstone Belt, South Africa; Isotopic, chemical, and structural characteristics of individual microstructures: Geochimica et Cosmochimica Acta, v. 71, p. 655-669, doi: 10.1016/j.gca.2006.09.029.
- Worms, J.-C., Lammer, H., Barucci, A., Beebe, R., Bibring, J.-P., Blamont, J., Blanc, M., Bonnet, R., Brucato, J.R., Chassefière, E., Coradini, A., Crawford, I., Ehrenfreund, P., Falcke, H., Gerzer, R., Grady, M., Grande, M., Haerendel, G., Horneck, G., Koch, B., Lobanov, A., Lopez-Moreno, J.J., Marco, R., Norsk, P., Rothery, D., Swings, J.-P., Tropea, C., Ulamec, S., Westall, F., and Zarnecki, J., 2009, ESSC-ESF Position Paper—Science-driven scenario for space exploration: Report from the European Space Sciences Committee (ESSC): Astrobiology, v. 9, no. 1, p. 23-41, doi: 10.1089/ast.2007.1226.
- Xiao, S., Hagadorn, J.W., Zhou, C., and Yuan, X., 2007, Rare helical spheroidal fossils from the Doushantuo Lagerstätte: Ediacaran animal embryos come of age?: Geology, v. 35, p. 115-118, doi: 10.1130/G23277A.1.

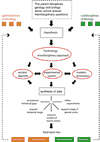 Figure 1
Figure 1 Figure 2
Figure 2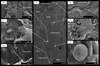 Figure 3
Figure 3