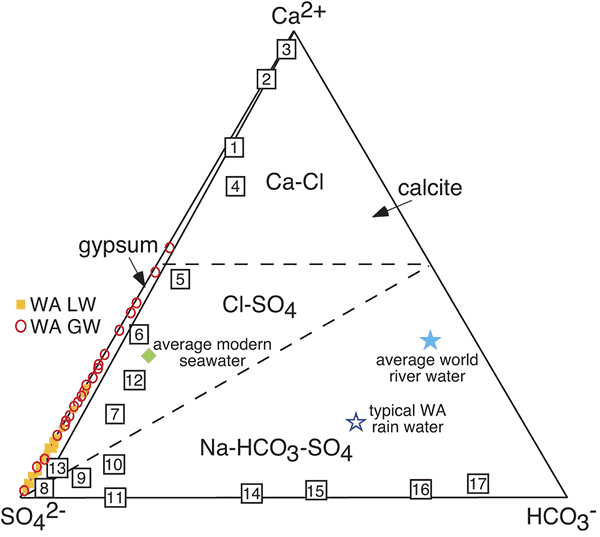
Figure 3.
Ternary diagram in equivalents for Ca-SO4-HCO3 showing the dominant carbonate and sulfate chemical divides that commonly occur as water evaporates. These types of diagrams are typically used to describe brine evolution in alkaline, neutral, or Ca-Cl type waters. The lack of HCO3− and calcite in the Western Australia (WA) acid lakes demonstrates that these systems are endmembers. WA lake waters are shown as orange squares and WA groundwaters are red circles (Bowen and Benison, 2009). Typical WA rain water values after Hingston and Gailitis (1976). Other examples of global extreme endmember saline systems are illustrated in comparison to the WA system. 1–3: Spring waters from China; 4–11: Surface brines from China lakes; average seawater and average river water; all after Lowenstein et al. (1989); 12: Salton Sea; 13: Great Salt Lake; 14: Deep Springs Lake; 15: Walker Lake; 16: Mono Lake; 17: Pyramid Lake; all after Spencer (2000).